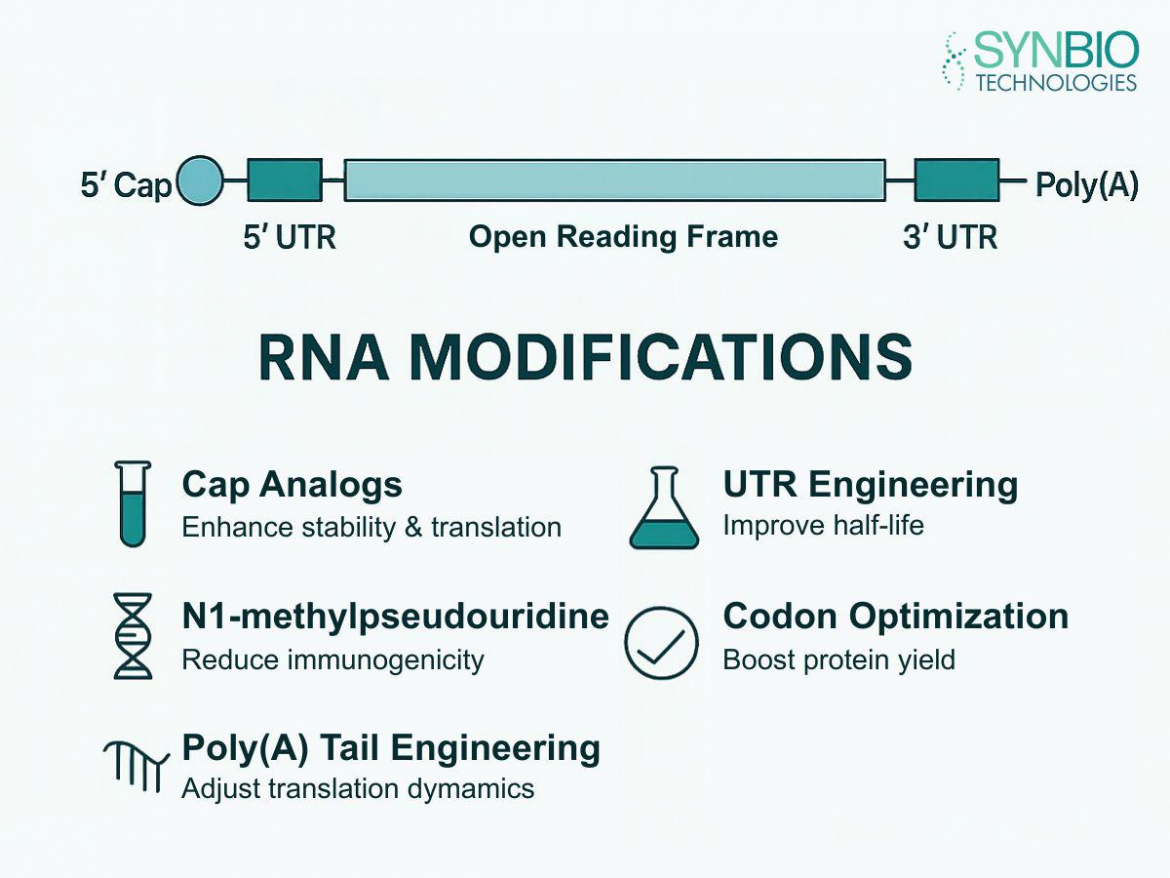
Synbio Technologies has become a reference point for researchers focusing on RNA production. As mRNA applications expand in therapeutics and diagnostics, understanding the critical quality control steps in RNA production is essential. They offer advanced solutions in RNA Synthesis that ensure consistent and reliable outcomes, providing a foundation for efficient gene expression studies and innovative research approaches.
Understanding the Importance of RNA Quality
Ensuring the integrity and purity of RNA is a vital aspect of mRNA production. RNA Synthesis involves multiple stages, from transcription to purification, each requiring careful monitoring. They implement rigorous checks to assess nucleotide composition, sequence fidelity, and absence of contaminants. These quality control steps prevent errors in downstream applications, making it possible to produce functional RNA molecules suitable for both research and clinical purposes. Additionally, maintaining RNA stability during storage and handling is another key consideration, as degradation can compromise experimental results. Proper handling and verification at each stage help mitigate risks and maintain high-quality outcomes.
Monitoring RNA Synthesis from DNA Template
A crucial aspect in mRNA production is RNA synthesis from DNA template. This process starts with a carefully designed DNA template that guides the transcription reaction. They emphasize the need for high-quality templates and precise enzyme selection to ensure accurate transcription. Continuous monitoring during this stage helps detect incomplete transcripts, ensuring the RNA products maintain the intended sequence and functionality. Furthermore, they incorporate real-time assessment techniques to detect potential errors or anomalies early in the process, which supports consistent production and reduces the likelihood of batch failures.
Purification and Validation Processes
After transcription, purification is essential to remove residual enzymes, nucleotides, and other by-products. They utilize methods that enhance the purity and stability of the RNA while preserving its biological activity. Validation steps, including electrophoresis and spectrophotometric analysis, confirm the quality of the RNA. These processes are integral to maintaining the reproducibility and reliability of RNA Synthesis, ensuring that researchers receive high-quality material for experimental and therapeutic use. They also recommend periodic assessment of RNA integrity using additional analytical methods to provide a comprehensive understanding of RNA quality, which is especially important for clinical applications where precise dosing and consistency are required.
Conclusion: Ensuring Reliable mRNA Production
In conclusion, meticulous attention to quality control is fundamental in RNA synthesis from DNA template. Synbio Technologies provides comprehensive support through advanced protocols and monitoring systems that uphold the integrity of RNA. By focusing on template quality, transcription accuracy, purification, and validation, they help ensure that mRNA products meet the stringent requirements of modern research and therapeutic applications. Adopting these systematic quality control measures allows laboratories to produce reliable RNA material, contributing to successful experiments and advancing the development of RNA-based treatments and vaccines.